Background:
RCHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) has been the predominant regimen for treatment of aggressive large B-cell lymphomas (LBCL). In the CALGB 50303 trial, REPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) was associated with similar efficacy but increased toxicity compared to RCHOP, however pts with higher risk disease by International Prognostic Index (IPI) had longer progression-free survival (PFS) with REPOCH therapy in the subgroup analysis ( J Clin Oncol, 2019). Providers may be hesitant to use REPOCH therapy in older, frail patients (pts) with high-risk LBCL due to concern for increased toxicity. One study demonstrated that empirically dose-reduced REPOCH (30-50% dose reduction by age group) was effective and well-tolerated in an elderly population ( Ann Hematol, 2018).A separate study showed significantly reduced neuropathy rates without compromised efficacy when vincristine doses in the REPOCH regimen were capped at 0.5 mg/d compared to 0.4 mg/m 2/d ( Leukemia & Lymphoma, 2019). At our institution, vincristine doses are capped at 0.4 mg/d for all pts, and REPOCH dosing is not empirically dose-reduced based for age alone. The aim of this study is to provide additional insights on the safety and efficacy of REPOCH in elderly pts diagnosed with LBCL with a routine vincristine dose cap of 0.4 mg/day (“REPOCH 0.4”).
Methods:
In this single cohort study, pts with newly diagnosed LBCL that received dose-adjusted REPOCH 0.4 at a single institution from August 2015 to October 2020 were retrospectively reviewed. Pts were excluded if they received fewer than 2 cycles, had CNS disease, transferred to another facility during treatment, or were lost-to-follow-up. Primary endpoints included adverse effects (AEs), overall response rates (ORR) via Lugano staging at end of treatment, 2y PFS, and overall survival (OS). Survival endpoints were estimated via Kaplan-Meier survival analysis. Secondary endpoints included CNS relapse rates and percentage of pts electing for hospice during therapy. Outcomes were descriptively compared to the historical cohort (N=241) in the CALGB 50303 trial receiving R-REPOCH without a vincristine dose cap ( J Clin Oncol, 2019) .
Results:
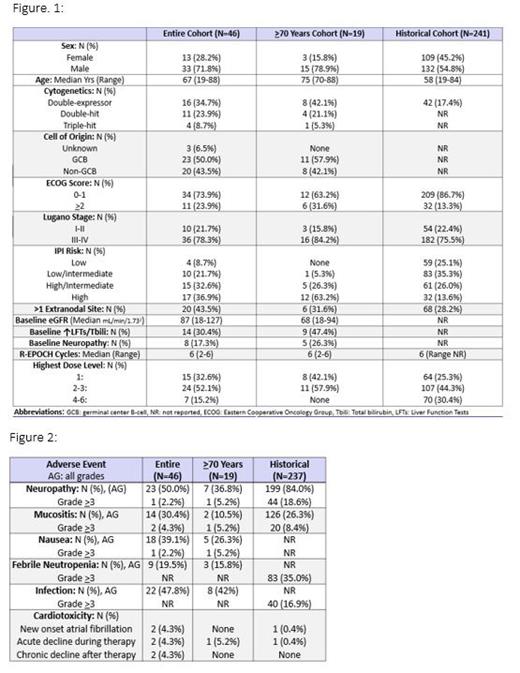
Forty-six pts were eligible for analysis (“entire cohort”), 19 of whom were 70 years or older (“70+ cohort”, figure 1). Median age in the entire and 70+ cohort was 67 and 75 years respectively. In the entire cohort, 23.9% of pts were double-hit, 43.5% had a non-germinal center phenotype, and 78.3% had stage III-IV disease. The median number of REPOCH 0.4 cycles received in both the entire and 70+ cohort was 6. In the 70+ cohort, 57.9% of pts were escalated to a dose level (DL) of 2 or 3 with no pts escalated beyond DL 3. Fifty-eight percent of pts in the entire cohort received intrathecal CNS prophylaxis and all pts received pegfilgrastim support. Pts in the entire cohort were older and higher-risk in comparison to the historical cohort as evidenced by IPI risk and double-expressor status. In the entire cohort, REPOCH 0.4 was well-tolerated (figure 2) with grade 3+ neuropathy rates of only 2.2% (5.2% in the 70+ cohort). Febrile neutropenia (all grades) occurred in 19.5% and 15.8% of the entire cohort and 70+ cohort respectively. Only 1 patient in the 70+ cohort experienced acute ejection fraction (EF) decline during therapy. ORR (complete and partial response) in the entire and 70+ cohort were 80% and 74% respectively. Complete response (CR) rates in the entire and 70+ cohort were both 63%. 2y PFS in the entire cohort was 68.3% (median not reached), and the 2y OS in the entire cohort was 82.9% (median not reached) which compares favorably to the 2y OS of 86.5% in the historical cohort. Overall, only 7% of pts elected for hospice and only 4% experienced CNS relapse.
Conclusion:
Pts with LBCL 70+ years tolerated dose-adjusted REPOCH 0.4 mg/d similarly to the entire cohort of all ages indicating that empiric dose reduction for age alone isn't necessary. CR rates were similar (63%) with pts 70+ years compared to pts in the entire cohort of all ages. REPOCH 0.4 mg/d was observed to have numerically lower rates of grade 3 or higher neuropathy (2.2%) compared to vincristine 0.4 mg/m 2/d (8.4%, J Clin Oncol 2019) and vincristine 0.5 mg/d dosing strategies (4.5%, Leukemia & Lymphoma 2019) . To our knowledge, this is the first report of outcomes with REPOCH using a vincristine dose cap of 0.4 mg/d.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal